Until mid 2007 cavipes was misidentified as Coenobita violascens.
Common name: none
Latin Origins: Concave
Distribution: eastern parts of Africa [9] , the Philippines, China, Japan, Malaysia, Taiwan [11], Polynesia, and Micronesia.[5]
http://en.wikipedia.org/wiki/East_Africa#Geography_and_climate
http://jnto.org.au/travellers/climate-weather/
http://www.travelchinaguide.com/climate/
http://www.weather-and-climate.com/average-monthly-Rainfall-Temperature-Sunshine-in-French-Polynesia
Habitat: Near to the sea, mainly in sandy areas alongside sheltered lagoons.[10] Mangrove forests are the preferred home of the cavipes species where they seek shelter in mangrove trees. In north Mozambique they exist only in the mangrove forests. Travels over 100m from shore. [ 13] Forest dweller who returns to the sea as needed for water replenishment, foraging, mating, spawning.
Ecology:Mostly nocturnal, supratidal, terrestrial, in most regions prefers sea water in it’s shell, except colonies in Quirimba who are reported to prefer fresh water. [1] Prefers to enter and fill shell with seawater. [10]
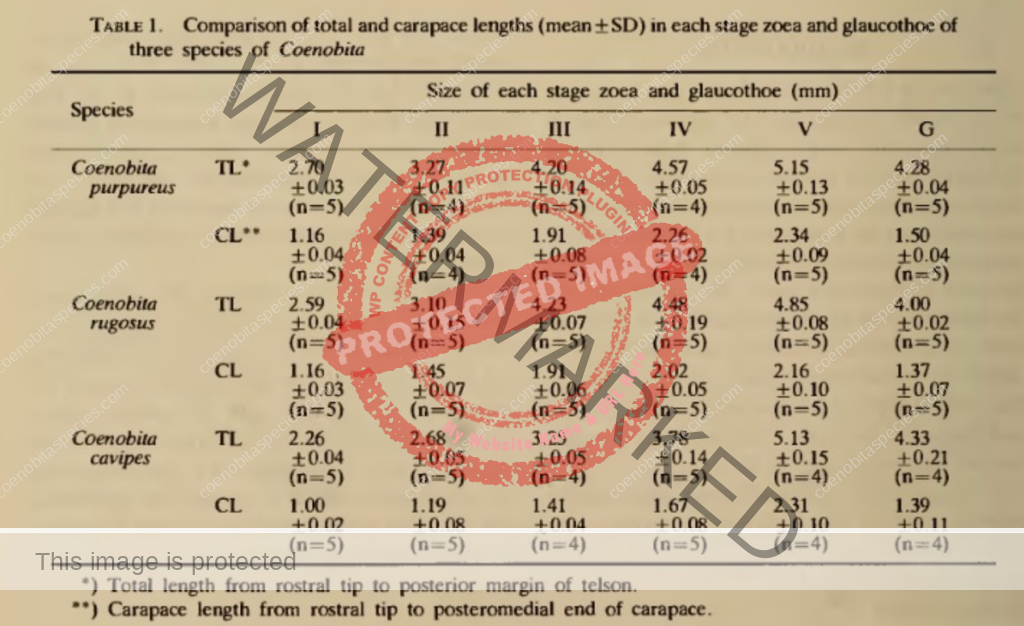
Lifecycle of larval stages: five zoeal stages and one glaucothoe state. The durations of the first to fifth zoeal stages were 7, 8, 5, 10, 15 days. [5]
Characteristics:
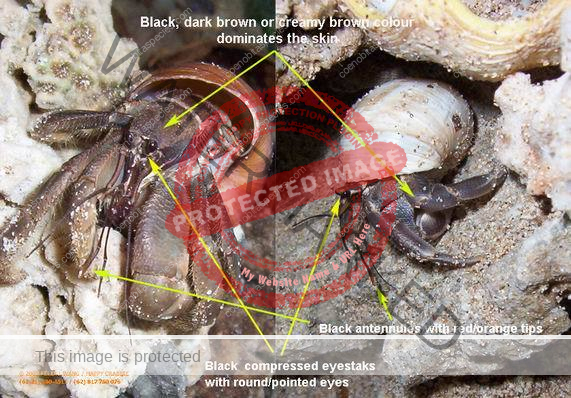
Coloration: Shield mottled brown and blue-gray. Shield longer than broad; rostrum triangular. Body color is basically brown with large pincer in a lighter color. Usually uses empty turbo shells, and occasionally part of a hard passion fruit.[6] Maximum reported shield length 16.0 mm.
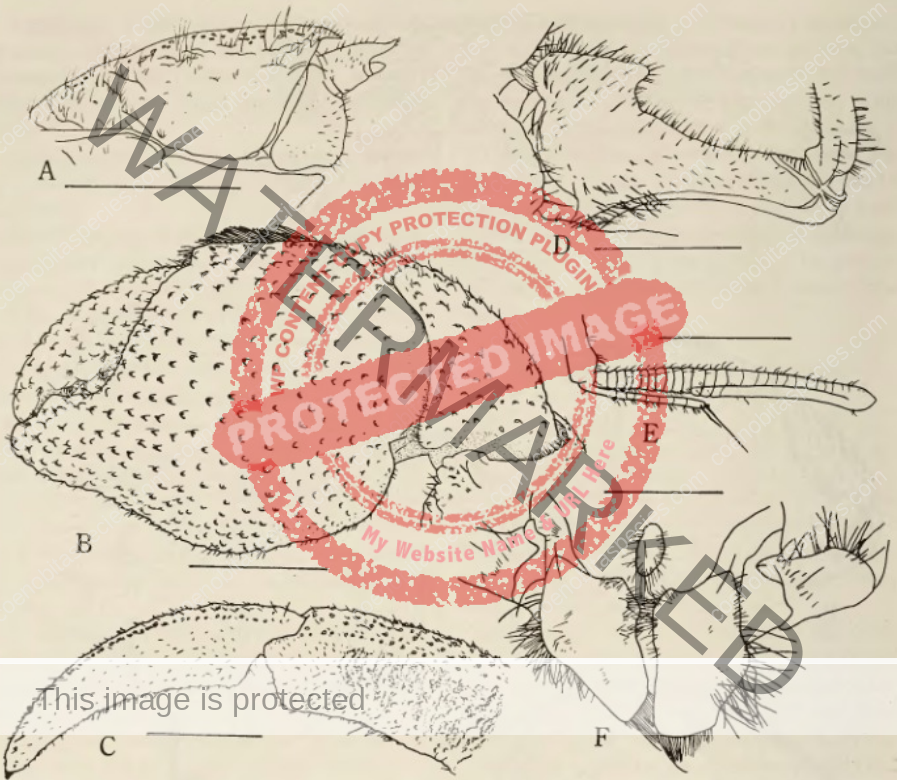
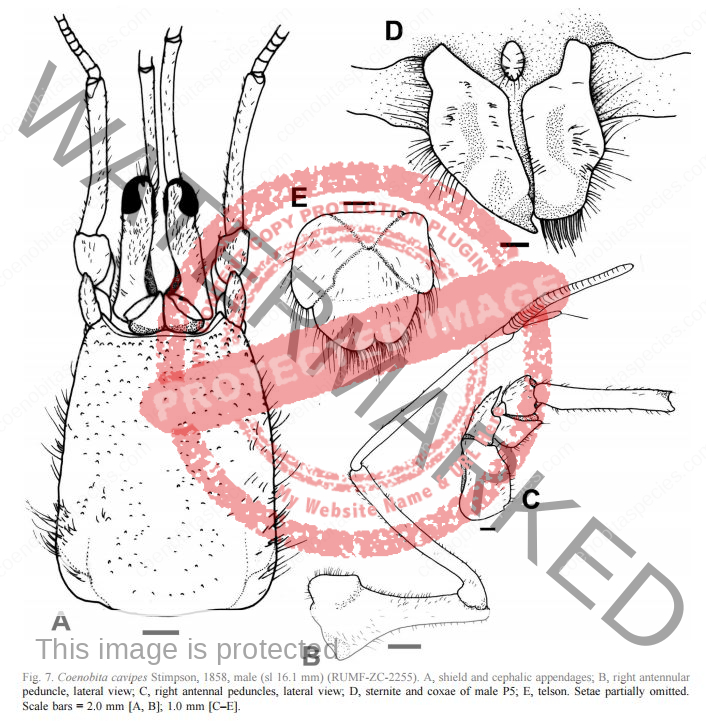
Eyes are elongated and the stalks are mostly black on adults. In juveniles, the lower portion of the stalk is black. Ocular peduncles generally white with broad dark band at midlength and dark brown patch and tinge of blue at base of cornea. Ocular peduncles half shield length or slightly less, corneas only faintly dilated; ocular acicles each with small submarginal spine. Antennular and antennal peduncles overreaching ocular peduncles.
Bottom section of second antenna are orange. Distal two segments of antennular peduncles each with broad band of reddish-brown and tinge of blue distally. Fifth segment of antennal peduncle transparent with brown mesial and lateral stripe. The anntenal aicicle is fused to the second segment of the peduncle.
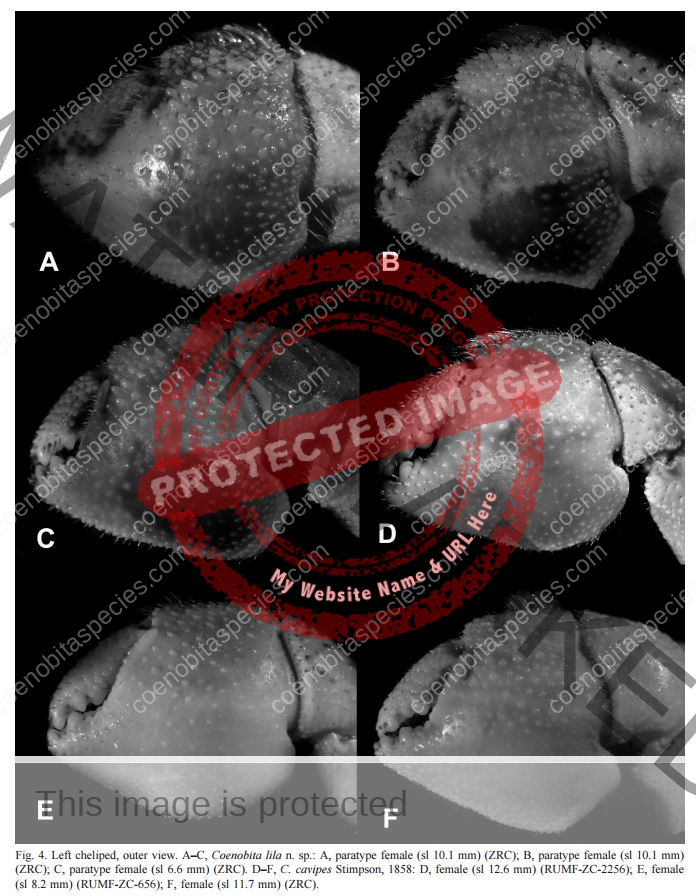
No stitch marks on large claw. Chelipeds generally brown with capsulate tubercles gray to blue-gray and spines brown to yellowish-brown; meri brown with spots of blue-gray. Chelipeds grossly unequal, both provided with numerous tufts of short or moderately short setae, often concealing armature, palms and carpi also with numerous capsulate setae on dorsal and lateral surfaces; dorsomesial and dorsolateral margins of palm of right cheliped each with row of small spines, dorsal surface with scattered small, often capsulate spines, one more prominent in midline; carpus with row of prominent spines on dorsomesial margin, dorsal surface with scattered capsulate tubercles and small spines, dorsolateral margin not delimited.
Left cheliped with chela elevated in midline but not forming distinct ridge, but armed with row of moderately large spines extending onto proximal half of fixed finger, dorsolateral surface with numerous capsulate tubercles, dorsomesial surface with scattered small spines; dorsomesial margin of carpus with row of large spines, dorsolateral margin only weakly delimited by few small spines.
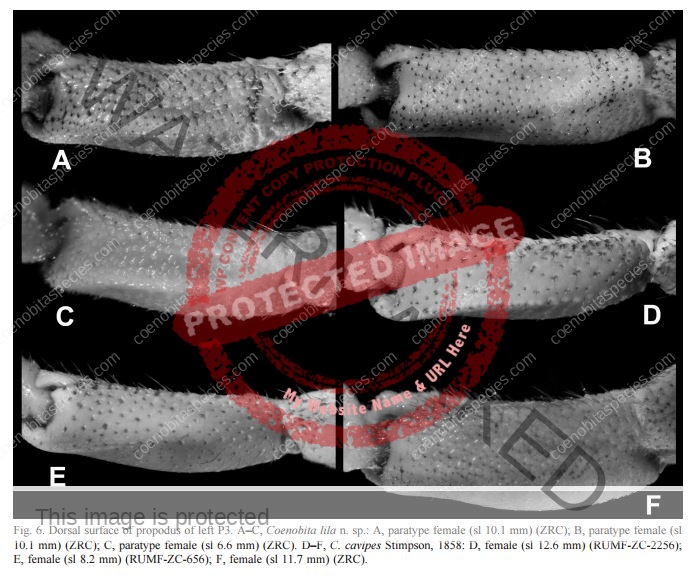
Third left leg is armored. Ambulatory legs similar; dactyls very slightly shorter to somewhat longer than propodi, ventral margins each with row of 7–10 prominent corneous spines; propodi unarmed but with transverse rows of long setae; carpi each with dorsodistal spine and tufts of long plumose setae. Telson with posterior lobes separated by small median cleft; terminal margins almost straight to very slightly concave, each with row of small spines, interspersed with minute spinules. Ambulatory legs with dactyls brown proximally, white distally; propodi reddish-brown proximally, blue-gray distally; carpi brown to reddish-brown, spotted with blue-gray; meri each brown proximally with large dorsal white patch and blue-gray distally with large dorsal brown patch.
Body mass 160g [10]
Common identifiers:
- Eyes – slightly elongated but stalks are comma shaped similar to clypeatus
- Shield – punctate, no setae or tubercles as in lila
- Large claw – no stitch marks
- Coloring – brown
- Chirps – yes
Behavior: Feed at low tide. [1]
Prefers to climb and shelter in mangrove trees, where it also will use water in holes to replenish shell water and shell swap, up to 30 individual hermit crabs have been witnessed congregating together in the same hole. They may stay dormant from 6 to 24 hours in these tree holes. Cavipes climbs high, up to 4 meters above high water. They are reported to travel as much as 100 meters in a couple of hours. Cavipes residing in mangrove forests are active throughout the day. Large crabs regularly immerse themselves in sea water. [1]
In the samples of C. cavipes collected from Hyakuna area and given to us by a pet trader, ovigerous females were found from 11 May to 2 August 1987. May produce two broods per season. [4]
The larval release of C. violascens (Japan) occurred around nighttime high tide during new moon periods, showing clear lunar and tidal rhythms. [14]
In contrast, the rhythm of larval release with regard to lunar and tidal phases is not clear for three congeners, C. rugosus, C. purpureus, and C. cavipes, all of which release larvae along the seacoast (Nakasone 2001). In these three species, larval release occurs mostly around high tide during both the full moon and new moon periods, but also occurs during other lunar phase periods; larval release occurs for 2 hours at spring tides, and become sporadic at neap tides. [14]
Diet: Cavipes has been viewed cannibalizing other smaller cavipes and rugosus. [1] Feed on human feces, fruit, rotting vegetation, dead fish and bird droppings. [3] Carrion(dead fish)[10] Fruits and Grains. [12] Animal feces, fallen leaves, seeds.
Preferred shells: thinoclavis sinesis, thais svigny, volema paradiscia, turbo cornoatus, and especially terebralia palustris and general tall spired shells. [2] Also turbo shells, Tonna and Achatina fulica

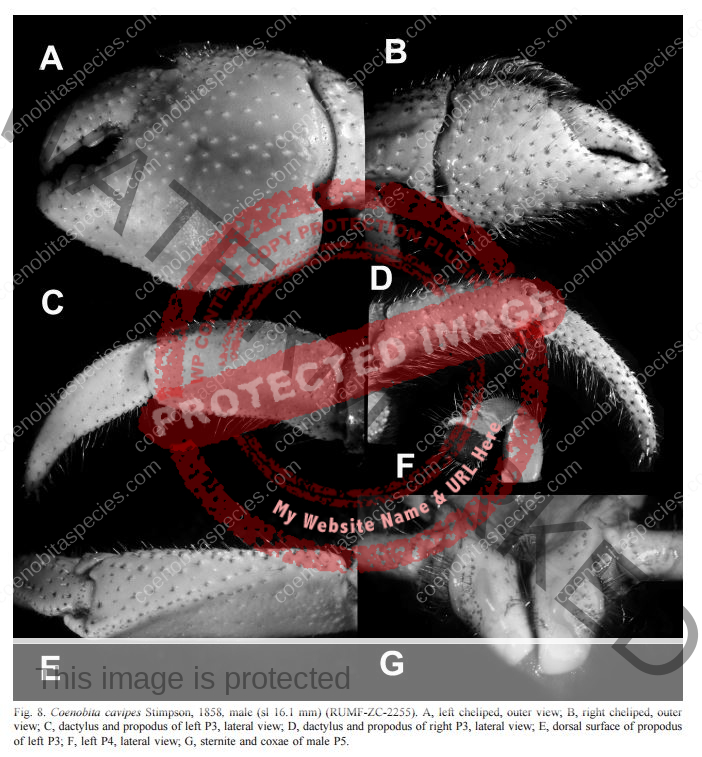
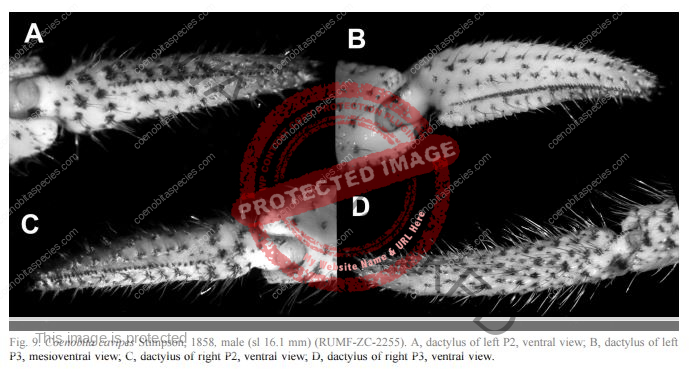
Coenobita cavipes


C. cavipes on Instagram
[insta-gallery id=”2″]
Photo Credit:
Stacy Griffith
Felix Wang
References:
1. David K. A. Barnes on Tree Migration 1997
2. David K.A. Barnes on Shell Characteristics and Utilization 1999
3. David K.A. Barnes on locally important food source 1997
4. Reproductive Biology of Three Land Hermit Crabs by Yukio Nakasone
5. Larval stages of Coenobita purpureus reared in the laboratory and survival rates and growth factors of three land hermit crab larvae by Y. Nakasone
6. Patsy A. McLaughlin, Tomoyuki Komai, Rafael Lemaitre & Dwi Listyo Rahayu (2010). Martyn E. Y. Low and S. H. Tan, ed. “Annotated checklist of anomuran decapod crustaceans of the world (exclusive of the Kiwaoidea and families Chirostylidae and Galatheidae of the Galatheoidea)” (PDF). Zootaxa. Suppl. 23: 5–107.
7. “Coenobita cavipes”. The common species of land hermit crabs.
8. Gerald McCormack (2007). “Coenobita cavipes, Unga Pūtua (AK). Brown Hermit-Land crab”. Cook Islands Biodiversity. Rarotonga: Cook Islands Natural Heritage Trust.
9. Coastal hermit crabs from Kenya, with a review and key to east African species by P. J. Reay and Janet Haig
10. Biology of the Land Crabs 1st Edition by Warren W. Burggren (Editor), Brian R. McMahon (Editor)
11. A new species of land hermit crab in the genus Coenobita latrielle -previously confused with C. cavipes by Rahayu, Shih, Ng
12. http://www.taipeitimes.com/News/taiwan/archives/2017/07/07/2003674074
13. What are the sympatric mechanisms for three species of terrestrial hermit crab (Coenobita rugosus, C. brevimanus, and C. cavipes) in coastal forests? Chia-Hsuan Hsu, Marinus L. Otte, Chi-Chang Liu, Jui-Yu Chou, Wei-Ta Fang
14. Larval release and associated tree climbing behavior of the land hermit crab Coenobita violascens Heller, 1862 Waturu Doi, Akira Mizutani, Hiroyoshi Kohno 2016