The family Coenobitidae is comprised of only two genera, Birgus and Coenobita. Both genera wear abandoned mollusk shells as protection after breaching land. Birgus latro only carries a protective shell for the first two years. Coenobita carry a protective shell for their entire life on land and will die from desiccation in less than two weeks without a shell. The shell allows land hermit crabs to carry life-sustaining water with them.
Scientific Classification of the Family Coenobitidae
- Kingdom: Animalia
- Phylum: Arthropoda
- Class: Malacostraca
- Order: Decapoda
- Family: Coenobitidae
- Genera:
- Coenobita
- Birgus
Taxonomic Classification of the Coenobitidae Family
The family Coenobitidae belongs to the order Decapoda and includes terrestrial hermit crabs, most notably the genus Coenobita. There are currently 21 accepted species of Coenobita. Below, you’ll find the full scientific classification of Coenobitidae, detailing where this unique group of land-dwelling crustaceans fits within the broader animal kingdom.
This page will cover the characteristics common to the Coenobitidae family with a focus on Coenobita. There exists physical differences among the Coenobita species. Birgus latro has their own page where all information will be compiled.
Origins of the name Coenobita:
Coen comes from the Greek word koinobion or koinos that means common.
The current English word coenobite refers to a monk who lives in a community.
Pronunciation of Coenobita: ‘seen-oh-bit-a
Coenobitadae Ecology
“Coenobitidae is a family of semi-terrestrial anomurans comprising land hermit crabs belonging to the genus Coenobita Latreille, 1829 and the coconut crab Birgus latro Linnaeus, 1767. Adult individuals of species of Coenobita are found in various terrestrial habitats. Some species inhabit areas close to shore (open beaches, rainforests, and mangroves), but others range from grassy areas and dry forests to altitudes of 900m and as far as 15km from the coast (Hartnoll, 1988; Wolcott, 1988). Ovigerous female coenobitid crabs approach the shore when their embryos are about to hatch because the larvae must spend their planktonic stage at sea (Hartnoll, 1988). Some species undertake breeding migrations with numerous crabs swarming coasts to release their larvae (De Wilde, 1973; Imafuku, 2001; Nieves-Rivera and Williams, 2003). The release of larvae is synchronized with lunar and tidal phases (Nakasone, 2001) as in other decapod crustaceans (Forward, 1987; Christy, 2011). Release sites are restricted to relatively narrow coastal areas (Wolcott, 1988; Imafuku, 2001).” [3]
Description of Coenobitids from Alcock 1905 [4]
COENOBITIDAE, Dana.
Coenobitida B, Dana, U. S. Expl. Exp., Crust., pt. I., 1852, pp. 432, 435; Stimpson, Proc. Ac. Nat. Sci. Philad, (1858) 1859, p. 232: Haswell, Cat. Austral. Crust., 1882, p. 159: Henderson, Challenger Anomura, 1888, p. 49: Stebbing, Hist. Crust., 1893, p. 155: Ortmann, in Bronn’s Thier Reich, Malaeostraca, 1900, p. 1146: Young, Stalk-eyed Crust. W. Indies, p. 357.
Carapace well calcified; either elongate, or greatly broadened in its posterior half. Rostrum either almost obsolete, or very prominent and completely concealing the ophthalmic somite. Thoracic sterna broad behind the chelipeds.
Abdomen either soft and spirally coiled in adaptation to a mollusk shell, or broad, symmetrical, dorsally well-calcified, and simply flexed.
Ophthalmic scales present. The antennular peduncle is of great length, the first joint deflexed and stout, the other two joints slender and cylindrical; the flagella compressed and truncated at tip. Antennal peduncle compressed, the acicle inconspicuous.
External maxillipeds approximated at base, the flagellum of the exopodite, as also of that of the 2nd maxillipeds, very much reduced.
Chelipeds massive, the left larger than the right. Legs of the 2nd and 3rd pairs elongate, those of the 4th and 5th pairs reduced, the 4th pair being cheliform or subcheliform, the 5th pair cheliform.
It is unusual for the male to possess recognizable: abdominal appendages other than the telson, but the female has a large biramous appendage on the left side of each of the abdominal somites 2, 3, and 4. The gills are phyllobranchise and are more or less subsidiary to respiration, which is largely effected by other means.
The Coenobitidae are land-hermits, visiting the sea occasionally, and in the case of the female periodically to hatch-off her eggs. They are characteristic of the tropical Indo-Pacific from East Africa to Panama, but the family is also represented in tropical parts of the Atlantic sea-board, both in America and West Africa.
The family contains but two genera Coenobita and Birqus.
In Coenobita the body is of the common Pagurine form, the carapace being elongate and the abdomen soft and spirally coiled, and the 4th and 5th thoracic legs have the usual Pagurine proportions; also the rostrum is obsolete.
Coenobita, Latr.
Coenobita. Latreille, Fam. Nat. du Regne Anim. 1S26, p. 276; and in Cuvier, Rcgne Anim. (2) IV., 1829, p. 77: Milne Edwards, Hist. Nat. Crust., 11. 1837, p. 238: De Haan, Faun. Japon. Crust., 1849, p. 203 ; Dana, U. S. Expl. Exp. Crust., pt. I., 1852, p. 435: Stimpson, Proc. Acad. Nat. Sci. Philad. (1858) 1859, p. 232; Hilgendorf in v. d. Decken s Reis. Ost-Afrika, Crust. 111. i. 1869. p. 97: Boas, Vid. Selsk. Skr., 6 Raek. Nat. o. math. Afd. 1. 2. 1880, pp. US, 190; Haswell, Cat. Austral. Crust., 1882, p. 160; Henderson, Challenger Anomura, 1888, p. 50: Bouvier, Bull. Soc. Philomath., Paris (8) II., 1889-90, pp. 143 and 194: Ortmann, Zool. Jahrb-, Syst., VI. 1892, p. 315, and in Bronn’s Thier Reich, Malacostraca, p. 1146: Stebbing, Hist. Crust., 1893, p. 159: Borradaile in Stanley Gardiner’s Fauna and Geography of the Maldive Islands, I. i., p. 69: Young, Stalk-eyed Crust. VV. Indies, &.C., 1900, p. 358.
Carapace elongate, more than ordinary contracted and compressed anteriorly—the contraction and compression involving all the appendages, from the eyes to the external maxillipeds—most strongly calcified anteriorly, but well calcified everywhere except in certain parts of the branchiostegites. Rostrum almost obsolete.
Eyestalks and ophthalmic scales juxtaposed, the former generally compressed: eyes terminal and lateral.
Antennular peduncles extremely long: the flagella compressed, rigid, and truncated at tip, the upper flagellum being much longer and broader than the lower. Antennal peduncles compressed, the acicle small and often fused with the 2nd joint, the flagellum long, coarse, and stiffish.
External maxillipeds juxtaposed at base: the exopodite of the first pair of maxillipeds non-flagellate; the flagella of the exopodites of the 2nd and 3rd maxillipeds short, hairy and non-segmented: the palp of the 1st maxillae non-flagellate.
Chelipeds unequal, the left being very much the stouter, all the joints short, broad, and clumsy-looking: the fingers move vertically, and have the extreme tip corneous or calcareous.
2nd and 3rd legs stout, not, or not much, longer than the larger cheliped. 4th pair of legs in a sort subcheliform, the dactylus being minute and the propodite forming a large suboval plate. 5th pair of legs cheliform, not shorter, and not much slenderer, than the 4th. The subterminal pavement of corneous imbricating granules is very well developed both on the 4th and 5th pair of legs and on the abdominal appendages that form the tail-fan.
The abdomen is soft, fleshy, and spirally coiled. Besides the appendages of the 6th abdominal somite (which are better developed on the left side than on the right) the female possesses 3 good-sized biramous appendages (somites 2—4) on the left side, which are either altogether absent, or are represented by rudiments (that are easily lost in spirit specimens} in the male.
The gills are phyllobranchise, and are 14 in number on either side arranged as in Pagurus, but the first four (the arthrobranchs of the external maxillipeds and chelipeds) are non-functional rudiments. The 10 functional gill-plumes are insufficient for respiration, and subsidiary respiration is carried on partly by the wall of the gill-chamber, and partly by the soft abdominal integument, which sometimes (anteriorly) grows out into excrescences for the purpose; for the members of the genus Coenobita, though they occasionally visit the sea, are ” land-crabs “, and often live at a distance from the shore.
The distribution of Coenobita is very like that of Clibanarius, to which genus (and to Calcinus) Coenobita is closely related. The majority of Coenobites are found m the coast lands and islands of the Indo-Pacific, from the Red Sea and East Africa to the eastern bounds of Polynesia. A few species extend to the Pacific coast of America, from California to Colombia and the Galapagos Is. One species occurs on the Atlantic seaboard of tropical America, from Brazil to Florida and the Bermudas; and two or three species are found in tropical West Africa. Two of the species found in West Africa have a most extensive range eastwards, one to Tahiti, the other to Panama.
The Coenobites form one of the most characteristic elements of the population of small tropical islands—especially of islands uninhabited by man —and can be observed to perfection (locally) in the smaller islets of the Andaman Archipelago and of the Laccadives. In the Andamans, though they swarm most in the belt of open jungle that fringes the beach, they are common enough in the depths of the forest.
They seem to prefer stout, heavy shells (e. g. Turbo and Nerita), but they are by no means fastidious about their tenement, and very large individuals, who find a difficulty in getting fitted, will make shift with the half of an empty coconut, or will even go unprotected.
They are very lively during rain, but they do not have much to do with the sea, though the females go to the sea to hatch-off their eggs, for the larvae are aquatic.
Though on occasion scavengers and carnivorous, they are chiefly vegetable-feeders, and will even climb trees in search of food.
A good account, both of the morphology and of the manner of life of the Coenobites, is contained in Borradaile’s Land Crustacean of Minikoi, published in Stanley Gardiner’s Fauna and Geography of the Maldive and Laccadive Archipelagoes.
Key to the Indian species of the genus Coenobita.
- Antennal acicle not fused with the 2nd joint of the peduncle eyestalks not strongly compressed: a brush of hairs on the inner surface of the right palm only — C. dypeatus
- Antennal acicle fused with the 2nd joint of the peduncle: eyestalks strongly compressed: a brush of hairs on the inner surface of both palms:
- An oblique file of upright laminar teeth (stridulating mechanism) on the upper part of the outer surface of the left palm:—
- Outer surface of propodite of third left leg flat, and separated from the anterior surface by a well-defined crest: coxa of fifth right leg of male moderately produced, more so than the left –C . rugosus.
- Outer surface of propodite of third left leg convex and not sharply separated from the anterior surface: coxa of fifth right leg of male produced into a long curved tube — C. perlatus,
- No stridulating mechanism on the left palm: the coxa: of the 5th legs are hardly more prominent in the male than they are in the female— C. cavipes.
The Habitat of Coenobita species
Coenobita are primarily found in tropical conditions near the sea. Some species are more terrestrial forest dwellers and only return to the sea for dispersing of their eggs, while other species are more dependent on the sea.
Land crab habitats are subject to temperature variation both on a daily and on a seasonal basis. Some habitats are extremely hot, such as the habitat of C. scaevola.
The equatorial zone in which Coenobita are found natively starts around the Tropic of Cancer and ends near Tropic of Capricorn.
View Distribution Map of Coenobitidae Distribution of hermit crab species map
The Diet of Coenobita species
All species of Coenobita are omnivorous scavengers. They play an important part in cleaning beaches and returning nutrients to the soil.
Growth and Molting
Coenobita dig underground and form a hollowed out cave in which to molt and consume their shed exoskeleton. Molting frequency varies as does length of molt. Generally speaking small hermit crabs molt frequently and quickly while very large hermit crabs molt much less often and their molts last many months. Several other variables can increase or decrease molting frequency. It is believed that 80-90% of arthropod deaths are molt related. Molting is a risky biological function.
For aquatic crabs, molting is a time of stress and mortality, resulting both from dangers inherent in the molt process itself and from the high risk of predation while the newly molted crabs are soft and relatively immobile. For land crabs there are added complications: The risk of desiccation is greater at this time, and there is the problem of obtaining the quantity of water needed for the postmolt increase in size, which must occur rapidly before the new integument hardens. Water is stored in the pericardial sac, located on the abdomen and in the shell. [1]
In crustaceans the frequency of molting is influenced by seasonal factors. In land crabs the seasonal availability of water is more significant than temperature, and molting may be restricted to the rainy season, as in B. latro. [1]
A dry substrate delays proecydysis (premolt) but does not prevent it. However, it does stop the uptake of water in premolt so that the usual size increase associated with a normal molt fails to occur. The more terrestrial species have the ability to absorb water from damp substrates. Molting is stimulated by conditions conducive to successful molting and reduced risk of predation. [1]
The Molt Cycle of Hermit Crabs by Stage:
Premolt (proecdysis) – The new integument is being laid down beneath the old, and stored energy reserves are being mobilized to enable the new structures to be formed. Calcium is being resorbed from the old integument and stored in the tissues. [1]
Molt (ecdysis) The old integument is shed, and the crab rapidly increases in size by the absorption of water. The process takes a most only a matter of hours. [1]
Postmolt (metecdysis) The new integument hardens using the stored calcium, plus additional supplies obtained from the water and food. Extra tissue is formed to build up the muscles and other structures in the new and larger body. [1]
Intermolt Formation of the new tissue is completed, and energy reserves are accumulated and stored pending the next molt. Final hardening of the integument is accomplished. During times of rapid growth the intermolt is relatively short and is known ad diecdysis. During slow growth, such as over the winter, a long intermolt termed an ancecdysis occurs. [1]
The rate of growth will depend upon two variables. One is the amount by which the size increases at a molt – the molt increment. This can vary from imperceptible amounts to as much as 80% carapace length, and generally the percentage increment falls with increasing size. The second variable is the time between molts- the intermolt period. This can vary from a few days to two or more years, and usually becomes longer with greater size. (Growth slows as size increases). In some crabs molting eventually ceases but this does not appear to be the case in land crabs. No data on age and grown rates in Coenobita has been found. [1] *
Coenobita Reproduction
Copulation occurs on land after the female hermit crab recovers from her molt, typically during the warmer months. Male hermit crabs will attempt to guard the female from other males. The larger, most dominant male typically wins the chance to mate. The male will turn the female so her shell opening is facing upwards. With his legs he will gently rock the shell and stroke her legs. In the C. brevimanus video below, mating was very calm. In captive C. rugosus a more animated mating ritual has been observed and recorded (Moa Lundberg). The female hermit crab must extend far enough out of her shell to allow access to her gonopores. The male places his coxae into the shell and on the openings, where he deposits his spermataphore. Actual mating can be seen in the video below.
The eggs are fertilized as they exit the gonorpores. Photo of hermit crab eggs being fertilized and extruded The female moves the eggs to the pleopods hidden in her shell. She will carry the eggs about a month. The eggs change color as they mature from orange to clear. The female hermit crab returns to the sea to disperse eggs into the water or along the tide line. This may occur for several nights. Female Coenobita can produce eggs more than once per season. This has been documented in captivity many times.
I captured a mating attempt between my Coenobita brevimanus on video:
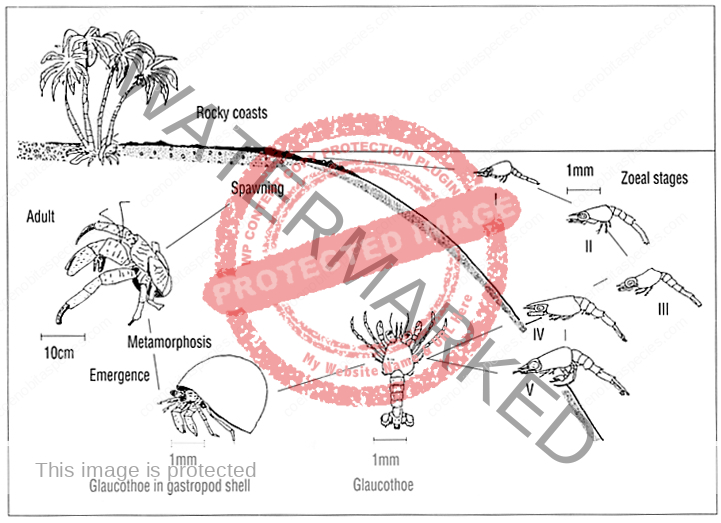
The first recorded description of Coenobita spawning and larval development
a) Spawning : The breeding season of Coenobita rugosus occurs once a year, i. e. at the end of June. It is a hot (about 25°-30°C.) and wet season. At that period all individuals of both sexes move toward the seashore, gather at a certain particular place, and incubating females enter the sea. Then at the end of July they move again toward the interior. The external reproductive organs and the secondary sexual characters of both sexes complete when their carapace length reaches 6 mm. And it has been found that a female with 8 mm carapace length was already incubating. Thus the animal continues its growth’ after reaching maturity.
The largest ones I have secured measure 32 mm (male), and 42 mm (female), that is, about five times the length when the animal began reproduction. Although the number of eggs carried differs largely according to the size of the mother, the eggs are almost uniform in size. In the female the second, third and fourth abdominal segments are provided on the left side with biramous pleopods, covered with hairs.
The eggs are retained by the female throughout their entire development. An incubating female (Pl. 2, fig. 1) protected by a spiral shell, can bear dryness for a considerable time. The male genital opening is on the
coxopodite of the fifth thoracic leg of each side. The portion of the joint which bears the opening is prolonged into a penis-like process. Its shape and size differ between the two sides. Either side projection equally juts out the spermatophore band. In it is spermatophore (fig. 3, sp) which contains spermatozoa grouped in a row. Presumably the eggs are fertilized by means of the spermatophore soon after being laid.
b) Development : The egg (fig. 2) is oval in shape and measures 0.73 mm x 0.56 mm. It has a reddish brown colour, glassy, and is covered by a double thin membrane which is strong and transparent. The zoea-egg – hatches out immediately at a stimulus exerted by seawater, and the zoea (fig. 4) becomes free. One can rear the hatched zoea by means of supplying oxygen with H202 solution in a vessel filled with sea-water of about 29°C. The zoea shows phototaxis when it is hatched. It always stands upside down perpendicularly, or makes a circular movement very smoothly, turning the tips of the telson ahead. ,The zoea (figs. 5, 6) posseses as usual the following appendages : the first and second antennae (fig. 7), mandibles (fig. 8 b), the first and second maxillae (fig. 8 c, d), and two pairs of biramous swimming maxillipedes and rudimental third maxillipedes (Pl. 2, fig. 9 ; Pl. 3, fig. 10). The carapace has a pointed rostral spine. The abdomen consists of six segments. The sixth abdominal segment constitutes the telson. It has five pairs of feathered bristles. And in addition to them, it has one short spine and one sensory hair on each side. The abdominal segments, except the first and sixth, have a spine behind, and the fifth has also a spine on each side. [9]
I could not collect any specimen of the metazoea stage. But I succeeded to find on a beach at low tide a glaucothoe which was already lodging in a spiral shell (Pl. 3, fig. 11). It showed a perfect symmetry of body. The abdomen has six segments and a telson. Each of the second to fifth abdominal segments has a pair of biramous pleopods (fig. 13), and the sixth abdominal segment has a pair of large uropods (fig. 14 a). On these abdominal appendages there grow feathered bristles. The asymmetrical structure first appears in the adolescent stage (figs. 14b; 15). Now the abdominal segmentation is lost, the pleopodes almost disappear, and the organization is completed as a hermit crab. But the external reproductive organs are not yet developed. [9]
Coenobita clypeatus spawning on Mona Island Account
In synchrony with the lunar cycle and tides, C. clypeatus travels many meters to spawn in the sea, a similar procedure as practiced by G. natalis. The season of migration and spawning of C. clypeatus in Playas Sardinera (f g. 1B) and Uvero (f g. 1C), corresponds to August or early September. This happens when
the moon phase is close to crescent moon (lunar cycle). The results of this study agree with the previous observations of Provenzano (1962), Erdman (1973), and Wiewandt (1975), in which the migrations occur in August or early September in connection with the lunar cycle and the tides. However, Provenzano reported
that the spawning occurred during the new moon, while Erdman and Wiewandt considered that it usually occurred during the crescent moon. Most recorded spawnings of C. clypeatus on Mona Island occurred during the crescent moon. [8]
Coenobitidae zoea pass through 2-7 stages, which varies by species and may be influenced by geography and temperature. C. variabilis has only two stages and C. scaevola has the most with seven stages.
The final stage is called Glaucothoe or Megalopa. The megalopa will take their first shell and exit the sea. The megalopa digs into the wet sand and molts, completing it’s metamorphosis into a true land hermit crab.
Ion and Water Balance in Coenobita species
Osmoregulation is the process of maintaining salt and water balance (osmotic balance) across membranes within the body.
In Birgus, which has probably made the largest and most successful step onto land for the decapod Crustacea, interesting behavioral mechanisms have contributed markedly to this success: A. It can moisten its respiratory membranes by its appendages, thus assuring adequate gaseous exchange. This it can do from small puddles of water without immersing itself. Such a behavior has not been observed nor reported among the semi-terrestrial crabs. Pachygrapsus must have sufficient water to immerse itself in order to live indefinitely. B. It can drink water without having to immerse itself. Thus it can be assured of adequate fluids from small, shallow sources. C. It can control the osmotic pressure of its body fluids by selecting among salt solutions the proper amount of salt. This means that not only is the animal precisely “aware” of its body fluid concentrations, but is also “‘aware” of a precise means by which it can keep such concentrations constant. [6]
Respiration
Respiration occurs through a modified gill, branchial chamber and an abdominal lung. Land hermit crabs breathe air on land but require a minimum relative humidity of 70% to keep the gills moist and access to fresh and brackish or sea water to replenish their shell water and adjust salinity. Once land hermit crabs leave the ocean they exist primarily on land, returning to the water to replenish shell water or spawn their eggs. It is possible for them to drown if under water too long. Pet land hermit crabs have been observed spending several hours under water intentionally (without drowning).
Gills
Gills that are primarily modified for osmoregulation will be less efficient in gas exchange. However, the development of alternative sites for gas exchange in air-breathing crustaceans has freed the gills for other functions without compromising the overall efficiency of gas exchange in the animals. Thus the pattern of gill function may vary within or between the lamellae and/or between gills, with either the anterior or posterior gills being essentially modified for ion-regulation. In C. perlatus, the posterior gills appeared to be primarily involved in gas exchange, whilst the gills of C. rugosus (examined by Harms, 1932) appear to be modified for ion regulation (however, it is not known which gills were studied by Harms). Further structural studies are needed to clearly identify the primary role of the different gills in the Coenobitidae.
The gills of the air-breathing coenobitids have a reduced surface area (fewer gills and fewer and smaller lamellae) and an increased blood/gas diffusion distance (thicker cuticle and epithelium) compared to aquatic hermit crabs and both these factors would tend to reduce the efficiency of oxygen uptake. Indeed the gills of Birgus are ineffective in O2 uptake (Greenaway et al., 1988). This suggests that the gills are no longer the primary site of gas exchange, although they may still have an important role in the excretion of carbon dioxide (Greenaway et al., 1988; Morris and Greenaway 1990). Clearly, with reduced branchial function, the development of alternative respiratory sites is essential for terrestrial coenobitids. [7]
Branchial chamber
The branchial chambers of Coenobita are small and narrow and are occupied almost entirely by the large posterior gills and pericardial sacs, which lie directly against the branchiostegal wall and thus further reduce the surface area of the branchiostegites available for gas exchange. Clearly, the branchiostegites of Coenobita are modified for aerial gas exchange but to a much lesser extent than in B. latro where the branchial chambers are expanded and the lung surface is greatly elaborated.
As discussed above, the branchiostegites of the coenobitids are better adapted for gas exchange than those of the pagurids. However, their gas exchange capacity is limited by their relatively small surface area and anatomically they are not as effectively organised as those of Birgus or some of the air-breathing brachyurans (von Raben, 1934; Greenaway and Farrelly, 1984; Farrelly and Greenaway, 1987, 1993). Although both branchiostegites and gills provide functional respiratory surfaces, their gas exchange capacity
seems insufficient to sustain the total respiratory needs of the animals and this has resulted in selective pressure favouring the development of an entirely new respiratory organ: the abdominal lung. This organ was first described by Bouvier (1890a) and then later by Borradaile (1903) and Harms (1932). Much of this early work was based on pericardial injections of ink and subsequent dissection and while the basic anatomical detail reported was correct, fundamental errors of interpretation were made in regard to the anatomy of the abdominal veins and the return of haemolymph from the abdominal lung to the pericardium. [7]
Abdominal Lung
It is clear from corrosion casts that the dense vascular network in the dorsal abdominal integument of Coenobita is supplied with venous blood from both the thorax and the abdomen. Blood from the thoracic ventral sinus flows posteriorly in two large afferent vessels that run down either side of the abdomen. Venous blood from the abdomen originates from the extensive dorsal abdominal sinus. Furthermore, the afferent veins, which lie on top of the dorsal abdominal sinus, join with the main abdominal sinus along their ventral edge, and thus are very closely connected. The dorsal abdominal sinus also gives rise to several long, thin channels that run parallel to the large lateral afferent vessels. These longitudinal channels branch laterally to form an afferent network of cross-channels. The afferent cross-channels feed the exchange lacunae that line the walls of the respiratory grooves. The lacunae in turn connect to an extensive efferent network of anastomosing cross-vessels that carry oxygenated blood to the outer efferent abdominal veins. These efferent veins run forward into the thorax where they supply the afferent vessel of the posterior gills on either side. Oxygenated blood from the abdomen must, therefore, pass through the posterior gills before it is returned to the pericardium via the branchiopericardial veins. Thus the abdominal lung functions in series with the gills (Fig. 12(C)–(E)) and not in parallel as reported in the earlier literature. [7]
We conclude that the presence of a protective mollusc shell in the terrestrial hermit crabs has favoured the evolution of an abdominal lung whilst in its absence a branchiostegal lung has been developed. Thus Coenobita, which has retained a shell, has a very limited branchiostegal lung (growth restricted by shell) but has developed a complex abdominal lung. Birgus, which discards its shell after its juvenile stages, has calcified and thickened its abdominal integument to give protection and reduce evaporative water loss, (thereby losing abdominal lung function), but has compensated by enlarging and developing its branchiostegal lungs. Both Coenobita and Birgus have extremely reduced gills. Increasing terrestrialisation in Coenobita is reflected by an increase in the number of segments involved with the abdominal lung, thus the more coastal species, C. perlatus and C. rugosus both utilize three segments, while in the more terrestrial C. brevimanus five abdominal segments have been incorporated, greatly increasing the surface area available for gas exchange. However, Birgus by giving up its protective shell has achieved even greater independence from the sea and must be regarded as having colonized land more successfully than Coenobita, in which mobility and growth are still restricted by a shell. [7]
Additional Reading
** Photo of Coenobita clypeatus eggs being fertilized
References:
1. Biology of the Land Crabs Warren W. Burggren and Brain R. McMahon
2. Hermit crabs : everything about anatomy, ecology, purchasing, feeding, housing, behavior, and illness Sue Fox
3. Larval Release and Associated Tree-Climbing Behavior of the Land Hermit Crab Coenobita ViolascensHeller, 1862 (Anomura: Coenobitidae) Journal of Crustacean Biology, Volume 36, Issue 3, 1 May 2016, Pages 279–286,
4. CATALOGUE OF THE INDIAN DECAPOD CRUSTACEA IN THE COLLECTION OF THE INDIAN MUSEUM. PART II. ANOMURA. FASCICULUS I. PAGURIDES. BY A. ALCOCK, M.B., LL.D., F.RS., CLE.
5. Wikipedia https://en.wikipedia.org/wiki/Crustacean
6. Aspects of Osmotic Regulation in Crabs Showing the Terrestrial Habit Author(s): Warren J. Gross Source: The American Naturalist, Vol. 89, No. 847 (Jul. – Aug., 1955), pp. 205-222 Published by: The University of Chicago Press for The American Society of Naturalists
7. The morphology and vasculature of the respiratory organs of terrestrial hermit crabs (Coenobita and Birgus): gills, branchiostegal lungs and abdominal lungs C.A. Farrellya, P. Greenawayb,* a32 Tramway Pde. Beaumaris, Vic. 3193, Australia bSchool of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, N.S.W. 2052, Australia
8. Spawning Mona Islands
9. Spawning and development of the Coenobita rugosus Shimao Yamaguschi 1938
10. https://www.marinespecies.org/aphia.php?p=taxdetails&id=196140
11. http://www.mesa.edu.au/crustaceans/
*Carol Ormes purchased Jon, Kate and another Coenobita clypeatus in 1976. The unnamed hermit crab died almost immediately. Kate lived until 2011. At the time of this writing (2020) Jon is still alive and well living with Carol in Fort Meyers Florida. Jon is quite large and requires a massive shell.